Chromatographic Standard ≥99%
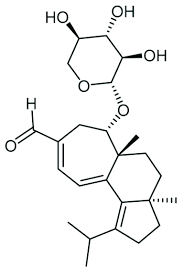
Erinacine A
Erinacine A Highest Quality Standard
for Scientific Research
STARTING FROM 100 EUR
Available immediately.
Bulk quantities available at favorable prices, send an inquiry through the form.